Chlorine was discovered in 1774 by extracting it from hydrochloric acid.
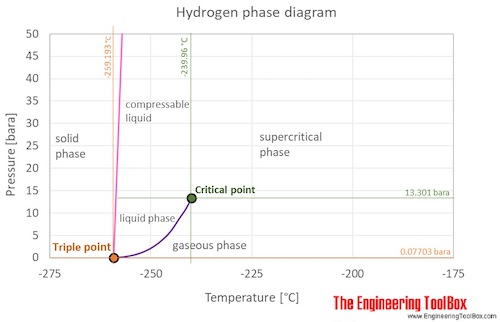
What state of matter is hydrogen at room temperature.
The study is detailed in the jan.
It is a gas and that would be the state of matter.
Regardless of the type of molecule matter normally exists as either a solid a liquid or a gas.
Elements that are gases at room temperature are all nonmetals such as he ar n 2 o 2 and so on.
Room temperature is a general term for what s considered a comfortable temperature.
It is a gas and that would be the state of matter.
Water molecules contain two atoms of hydrogen h and one atom of oxygen o and is chemically called h2o.
These weak bonds hold water molecules together for mere milliseconds which keeps water in a constantly liquid state at room temperature.
7 issue of the journal nature.
Metallic hydrogen is a phase of hydrogen in which it behaves like an electrical conductor this phase was predicted in 1935 on theoretical grounds by eugene wigner and hillard bell huntington.
Water is a liquid at room temperature because the hydrogen bonds within its construction are weak.
However hydrogen is not solid at room temperature.
Chlorine exhibits multiple oxidation states such as 1 1 3 5 and 7.
There are several patterns in the table above.
However hydrogen is not solid at room temperature.
Solid liquid gas and plasma.
Theoretical predictions also suggest liquid metallic hydrogen might also be a room temperature superconductor.
At high pressure and temperatures metallic hydrogen can exist as a liquid rather than a solid and researchers think it might be present in large quantities in the hot and gravitationally compressed.
Common gases at room temperature include both elements such as h 2 and o 2 and compounds such as co 2 and nh 3.
If it were a solid at room temperature then that would be the state of matter.
At room temperature it appears as a light green gas.
Compounds that are gases at room temperature are all covalent compounds such as co 2 so 2 and nh 3 that contain two or more.
In its elemental state it forms the diatomic molecule cl 2.